
OPEN DATA MICCAI 2025
Unlock Medical Machine Learning with Open Data
Daejeon Convention Centre - Daejeon, South Korea
WELCOME MESSAGE
Dear Researchers,
We are delighted to announce the second edition of Open Data at MICCAI 2025!
As the landscape of medical machine learning continues to evolve, access to diverse, representative, and inclusive datasets remains crucial for addressing global healthcare challenges effectively. The Open Data initiative aims to foster collaboration and innovation by promoting the sharing of medical imaging datasets.
Building on the foundation of the first edition, Open Data continues to emphasize underrepresented populations and diseases. Despite progress in medical imaging research and the availability of public datasets, critical gaps persist—particularly in regions such as South-East Asia. By highlighting datasets from underrepresented populations and diseases, we strive to reduce disparities in healthcare and promote inclusivity in machine learning research.
This year too, we plan to establish a dedicated repository to host publicly available high-quality medical imaging datasets, with a focus on underrepresented populations and diseases. Information regarding the repository structure and storage guidelines will be shared at a later stage. The initiative remains closely aligned with the principles of FAIR data and Data-centric AI, where data and its systematic engineering are at the forefront of model development. After all, there are no "good" models without "good" data.
MICCAI will be held on September 23-27, 2025, at the Daejon Convention Center, South Korea, and the Open Data event will run in parallel to the main track. Hence if you are visiting MICCAI, you can visit the Open Data event: no separate registration is required.
The presented datasets and related works will be selected through a peer-reviewed paper submission process. The MELBA journal will serve as the official journal for submissions from the MICCAI Open Data track.
We look forward to seeing you at the 2nd Open Data session at MICCAI 2025—welcome!
REPOSITORY
To maximize the impact of shared medical imaging datasets, we have established a set of clear guidelines for data uploading to ensure accessibility and usability across the research community. By following the instructions below, you can contribute to a well-organized, reusable, and collaborative open-data resource.
! Important ! All data—including imaging and clinical variables—must be anonymized. Any information that could link the data to a patient's identity or medical records must be removed.
-
Hosting and Accessibility
-
Data Organization and File Structure
-
Patient and Demographic Information
- To support fairness and bias analysis, please include anonymized patient demographics where available (eg. Age, Sex, Race/Ethnicity).
- Clearly indicate in your documentation which fields are provided and their format.
-
Task Definition and Labels
- Clearly define the intended task(s) for your dataset (e.g., segmentation, classification, detection).
- Indicate in your documentation: which files contain labels, the label format (e.g., masks, CSV annotations), any relevant class definitions or codes used.
-
Documentation Requirements
-
Naming and Consistency
- Use consistent naming conventions across the dataset.
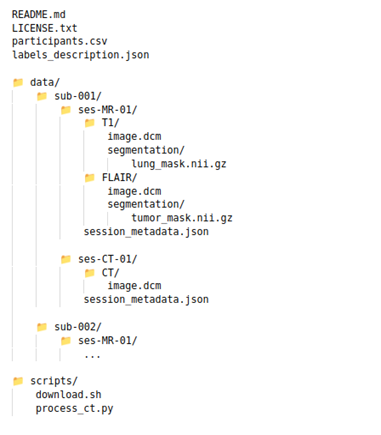
- Avoid ambiguous file names like 12345x6.nii, final.png. Prefer informative structure and naming like sub-001/ses-MR-01/T1/image.dcm or lung_mask.nii.gz.
Ideally, at the end of the upload your dataset would have a structure similar to the picture below:
TOPICS OF INTEREST
The main focus is on:
- New medical imaging datasets that encompass diverse demographics, ethnicities, and medical conditions. This year is especially focused on South-East Asia, but we also welcome datasets from other (underrepresented) populations or diseases.
- Updated or re-designed datasets based on previously publicly available data.
Additional topics include:
- Dataset collection and annotation techniques.
- Data augmentation strategies for improving dataset diversity.
- Ethical considerations in data sharing and privacy preservation.
- Applications of open data in medical image analysis, diagnosis, and treatment planning.
- Challenges and opportunities in accessing and utilizing underrepresented datasets.
IMPORTANT DATES
- Call for papers
- Now Open
- Paper submission deadline
- 23:59 CET,
June 16 June 30, 2025
- Review deadline
- 23:59 CET,
June 30 July 14, 2025
- Notification of acceptance
- 23:59 CET,
July 4 July 18, 2025
- Camera-ready paper submission
- 23:59 CET, July 25, 2025
SUBMISSION
We welcome submissions of papers presenting novel datasets, particularly those from South-East Asia and other underrepresented populations and diseases - including methodologies for the data collection and curation, and innovative approaches for utilizing them in medical imaging research.
PAPER SUBMISSION SITE
SCOPE
- Encourage and empower through an open repository the sharing and dissemination of open-access datasets to facilitate collaboration and reproducibility.
- Promote awareness and understanding of the importance of inclusivity and representative data in developing robust and equitable healthcare solutions.
- Facilitate networking opportunities among researchers, data custodians, and stakeholders interested in leveraging open data for medical machine learning.
CRITERIA
- In order to optimize the data upload process we request you to provide metadata about the dataset. Please use the DATA SUBMISSION FORM, and contact us for special requests.
Upon submission the data does not have to be uploaded yet, only the submission form has to be submitted. Before acceptance, the data has to be uploaded. If you have strong reasons why this is not possible for your dataset, please contact the Open Data Chairs to see if an exception can be made.
- Adhere to the FAIR data guidelines.
- Any associated code should be open source.
AUTHOR GUIDELINES
Listed below are important requirements, besides the above criteria, for preparing and submitting a manuscript to Open Data MICCAI 2025. Accepted papers will have the opportunity of oral presentation at the Open Data session, and will be invited for submission at the MELBA journal Resource track to be published in a special issue on Open Data MICCAI 2025.
-
Manuscript template: Submissions must be limited to a maximum of 8 pages for text, figures, and tables and up to 2 additional pages for references. You must follow the MELBA journal latex template for submissions available here (i.e., melba-sample.tex).
- NO cover letter addressed to MELBA is needed at this stage.
- Please follow the format of MELBA Resource manuscripts for Data Resources, available here.
- In addition to the above, we request that you include information on how the presented data adhere to the FAIR data principles; licensing, access procedures, and ethical considerations (including anonymization/pseudonymization practices); detailed descriptions of the (meta)data, including collection, equipment and acquisition protocols, data model and format, processing, curation, ground truth definition, and known errors/limitations; and comprehensive visual examples.
-
Dataset description: To ensure the quality and utility of shared medical imaging datasets, we have established the following minimal information requirements for data submissions:
-
Dataset Overview
- Title: A concise and descriptive title of the dataset.
- Abstract: A summary outlining the dataset's purpose, scope, and potential applications.
-
Data Acquisition Details
- Imaging Modality: Specify the type of imaging used (e.g., CT, MRI, pathology, microscopy).
-
Equipment Specifications
- Manufacturer and Model: Detail the imaging device's manufacturer, model, and any relevant technical characteristics (e.g. field strength for MRI)
- Acquisition Settings: Provide key parameters such as resolution, magnification, and imaging protocols.
-
Subject Information
- Demographics: Include anonymized data on age, sex, and relevant clinical information.
- Cohort Description: Describe the selection criteria, including inclusion and exclusion parameters.
-
Annotation and Segmentation
- Annotation Protocols: Detail the methods and standards used for annotations or segmentations, including any automatic tools used in the annotation process.
- Annotator Expertise: Specify the qualifications of individuals who performed the annotations.
- Inter-Observer Variability: If applicable, report measures of consistency among different annotators.
-
Data Format and Structure
- File Formats: List the formats of the image and annotation files (e.g., DICOM, TIFF, JPEG).
-
Supplementary materials: An upload link (of maximum two files) will be made available in your author console after you have created your submission. DO NOT append your supplementary material at the end of your main paper. As supplementary material you should upload:
- Any supporting information for the presented datasets that do not lie to the main manuscript requirements, e.g., additional visual examples.
- A print of the data submission form filled specifically for the presented data.
ACKNOWLEDGMENT
The Microsoft CMT service was used for managing the peer-reviewing process for this conference. This service was provided for free by Microsoft and they bore all expenses, including costs for Azure cloud services as well as for software development and support.
REVIEW GUIDELINES
This is a single-blind review process. For general guidelines on "What Makes a Good Review" please refer to the corresponding section in the MICCAI reviewer guidelines.
For this track, pay special attention on the Submission guidelines listed above - scope and criteria, summarized below (in order of priority):
- Data availability and adherence to the FAIR data principles.
- Licensing, potential use cases, and ethical considerations/approvals.
- Methods used for the data and meta-data creation/collection: from the methods and equipment used for the acquisition to final processing, and the AI-ready state of the dataset (cleaning, curation, possible suggested splits, etc.).
- Clarity of the dataset specifics.
- Dataset usage showcase(s) with evaluation results.
KEYNOTES
PROGRAM
PLEASE CLICK HERE FOR OPEN DATA 2025 PROGRAM
For updates and questions, you can find us on:
ORGANIZING COMMITTEE
- Martijn P.A. Starmans, PhD
- Assistant Professor AI for Integrated Diagnostics (AIID)
Dept. of Radiology & Nuclear Medicine, Dept. of Pathology
Erasmus University Medical Center, Rotterdam, the Netherlands
- Apostolia Tsirikoglou, PhD
- Research Specialist, AI for Breast Imaging
Dept. of Oncology-Pathology,
Karolinska Institutet, Sweden
- Namkug Kim, PhD
- Assistant Professor of Convergence Medicine
Asan Medical Center, Seoul, South Korea
University of Ulsan College of Medicine, Ulsan, South Korea
- Lidia Garrucho Moras
- PhD Candidate, EUCanImage AI Lead
Artificial Intelligence in Medicine Lab
University of Barcelona, Barcelona, Spain
- Kaouther Mouheb
- PhD Candidate, Medical Image Analysis and Federated Learning
Dept. of Radiology & Nuclear Medicine
Erasmus University Medical Center, Rotterdam, the Netherlands

